Abstract
Molecular monitoring of minimal residual disease (MRD) in Acute Myeloid Leukemia (AML) patients (pts) with mutated NPM1 (NPM1+) is a prognostic tool, not only for predicting disease relapse but also for its potential role to guide pre-emptive therapy in early-stage relapse (Ivey 2016). The utility of MRD detection by multiparameter flow-cytometry (MFC) in the setting of AML pts with a molecular marker is still unclear. We evaluated the prognostic significance of molecular and MFC MRD monitoring on pts outcome and the efficacy of relapse treatment in AML NPM1+ pts at a single Institution.
From Feb-2010 to Nov-2016, 172 consecutive AML pts eligible for intensive chemotherapy were evaluated. Sixty-eight pts were NPM1+ (39.5%) at diagnosis. The pts received ICE (idarubicine-ARAC-etoposide) induction therapy, followed by IC consolidation and 3 cycles of high-dose ARAC (HDC), according to the NILG AML00 protocol (Bassan 2003). Allogeneic stem cell transplant (HSCT) was considered in pts at relapse. Quantitative RT-PCR was performed to detect NPM1 mutation (Gorello 2006) on bone marrow (BM) and peripheral blood (PB) samples at diagnosis and at given time points (TP): complete remission (CR)-achieving (TP1), post-IC (TP2), and post-1st cycle of HDC (TP3). Eight colors MFC was performed on BM at diagnosis, and, in pts with a leukemia-associated immunophenotype (LAIP), at TP1 and TP2 as well. We used MRD cut-off values of 0.1% and 0.035% at TP1 and 0.035% at TP2 (Buccisano 2010), which we also validated (Chiarini 2017). Molecular relapse (mR) was defined as the detection of increasing levels of NPM1 transcript in 2 consecutive samples in absence of hematological relapse.
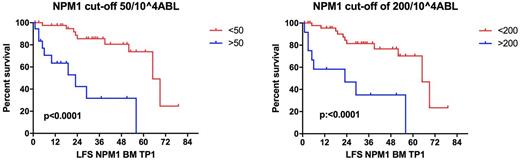
MRD by PCR and MFC was performed at all time points in 61 pts, who represent our study cohort. M/F was 1.25/1, median age was 56 years (27-74). K was normal in 54 (88%) pts, and 23 (38%) had the ITD-FLT3 mutation. Sixty pts (98%) achieved a CR after ICE and 1 pt after 2 cycles. Five pts (8%) experienced an early relapse after IC and did not undergo HDC. Twenty pts (32.7%) have relapsed after a median time from CR of 16.6 months (mo) (1-61.5): 9 were molecular relapses (mR)(median 22 mo, 13.6-61.5) and 11 hematological (hR)(median 5.7, 1-39.2). After a median follow-up of 33 mo (1-86), median survival was 61.5 mo, with a 5-year leukemia-free survival (LFS) and overall survival (OS) of 62% (+/-11% SE) and 71% (+/-8% SE), respectively. Age, NPM1 level at baseline, ITD-FLT3 mutation or abnormal k did not impact on relapse risk. We therefore evaluated NPM1 on BM and PB at different TP: a NPM1 positivity at TP3 on PB was associated to a higher risk of relapse (p 0.027). Quantitative NPM1 confirmed that a high level of NPM1 at TP3 on PB unfavorably impacts on disease relapse (p 0.007), and showed a negative predictive value of NPM1 level at TP1 on BM (p 0.008). Moreover, we found that NPM1 cut-offs of >50 and >200/10^4ABL were significantly associated with a higher risk of relapse (p 0.0066 and p 0.0002, respectively), also affecting LFS (Figure 1). To further identify pts at higher-risk of relapse, we monitored MRD by MFC. Forty-seven (77%) pts had a LAIP at diagnosis (CD34- in 39, 83%). Although a trend to a better LFS was seen for MRD negative pts at TP1 using 0.035% cut-off, MFC MRD monitoring did not significantly impact on OS and LFS. Relapse could be treated in 18/20 pts, including mR pts considering their high risk of hR (Kronke 2011). Of 9 pts in mR, 6 received non-intensive therapy (ni-T)(2 ATRA and 4 D-actinomycin, Falini 2015) for a median of 3 cycles, and 3 received intensive therapy (i-T). None of these pts progressed to hR and all 9 pts underwent HSCT, 7 being alive and in remission at 10 mo (5-26). Nine of 11 pts with hR were treated, 7 with i-T and 2 ni-T (Dactinomycin). Two CR were obtained (1 with ni-T). Five hR pts underwent HSCT, only 2 being alive and in remission at 10.4 and 22.7 mo, respectively.
Our study shows that PCR monitoring during treatment can identify pts at higher risk of relapse according to the transcript levels, and further studies on larger cohorts hopefully will help to understand its utility to guide the treatment approach. We could not demonstrate a prognostic advantage from the use of MRD monitoring by flow cytometry, possiby due to the small pts number. Finally, our results confirm that, in pts with NPM1+ AML, molecular MRD monitoring is of crucial importance in detecting relapse at an early-stage, allowing to use less toxic molecular-oriented treatments as a bridge to HSCT.
Rossi: Janssen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal